What is POC?
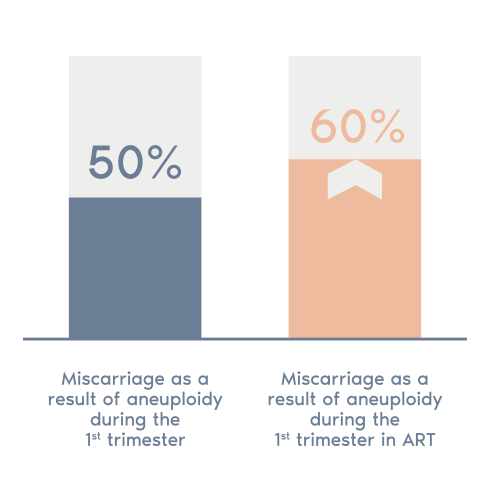
The POC test analyzes fetal tissues from a miscarriage to determine if the loss was the result of a chromosomal aneuploidy.
This test can provide you with important information about the possible causes of your patient’s miscarriage and help you determine the next steps for their fertility treatment.
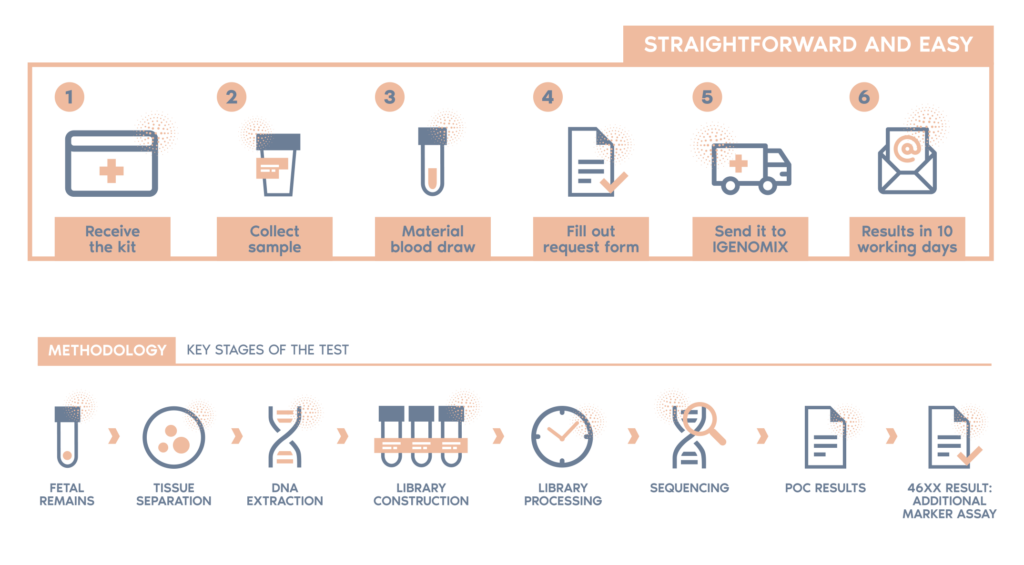
What is the procedure?