Why choose ERA®?
Launched in 2011, ERA was the first test of its kind, revolutionizing endometrial diagnostics and laying the foundation for personalized fertility treatment. By identifying a woman’s unique window of implantation, it enables precise embryo transfer timing to optimize IVF success. Backed by robust clinical evidence, ERA remains the most advanced and trusted endometrial receptivity test available.
1. Scientifically proven and well trusted
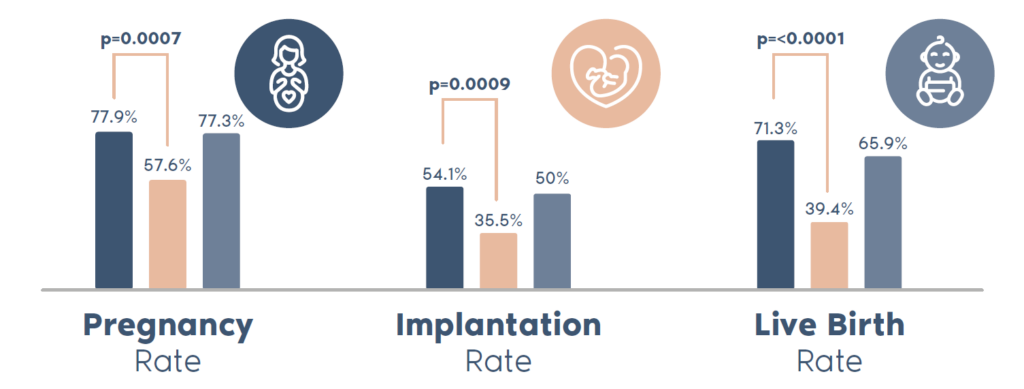
ERA is a proven solution for patients with RIF, powered by the most scientific publications and the highest clinical success rates compared to any other endometrial receptivity test in the market. In fact, ERA is the only endometrial receptivity test backed by an RCT*. This RCT, involving 320 patients with RIF, demonstrated significantly improved reproductive outcomes—including nearly double the live birth rates—for patients who used ERA in combination with PGT-A, compared to those who used PGT-A alone.
2. Precision timing for embryo transfer, maximizing your chances of success
Powered by AI, the ERA algorithm is not only based upon the endometrial biopsy data from over 200,000 women but integrates clinical outcomes from personalized embryo transfers guided by its results. Combining this with the most comprehensive gene panel available (248 genes), ERA delivers the most precise transcriptomic profiling and personalized embryo transfer timing—maximizing implantation success.
3. Comprehensive support & expertise
Backed by a global team of endometrial experts, ERA is the trusted endometrial receptivity test used by over 3,000 clinics worldwide. Our comprehensive support network includes world-class scientific advisors, dedicated endometrial specialists, genetic counsellors, and in-house clinicians—providing expert guidance to clinics and patients every step of the way.
* 2024 Barbakadze et al. Cureus 16(6): e62949. DOI 10.7759/cureus.62949