What is NACE?
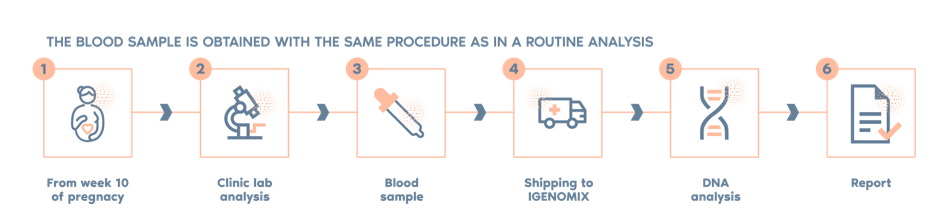
NACE is a non-invasive prenatal screening test for the most frequent chromosomal abnormalities. The test is non-invasive, meaning that there is no risk to the fetus.
A simple blood draw from the mother allows free fetal DNA circulating in the maternal bloodstream to be detected via next-generation sequencing (NGS) technology and our proprietary bioinformatic analysis tool.